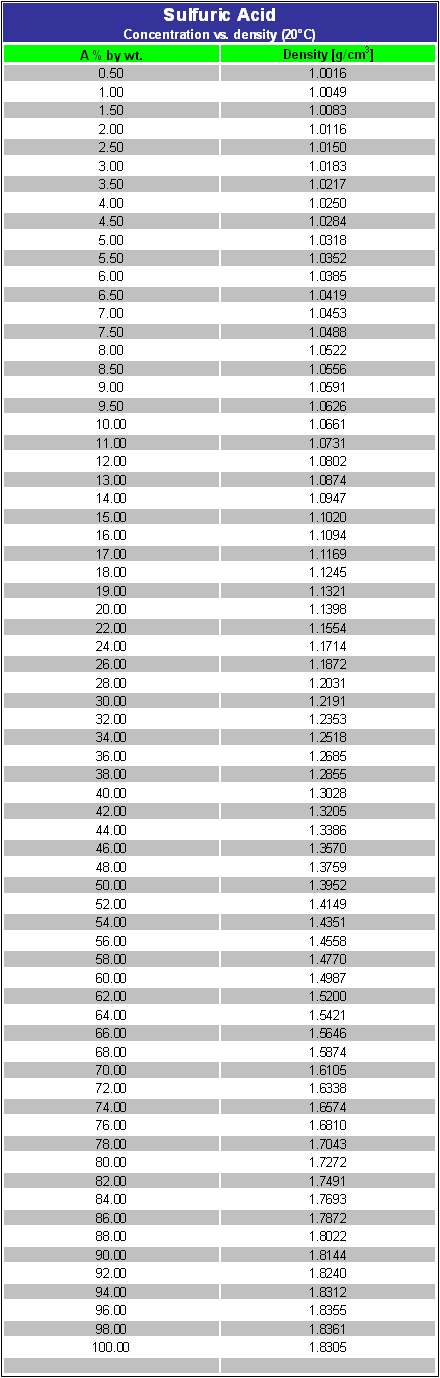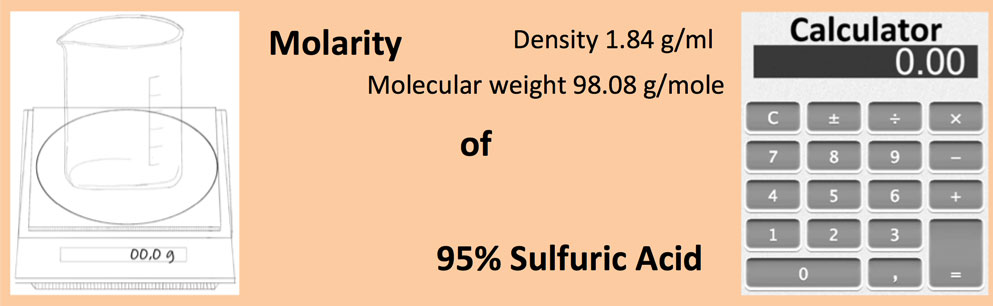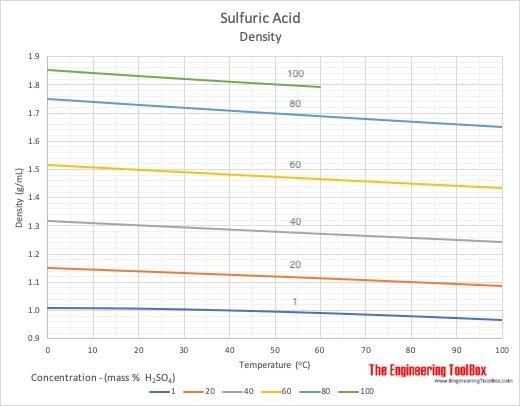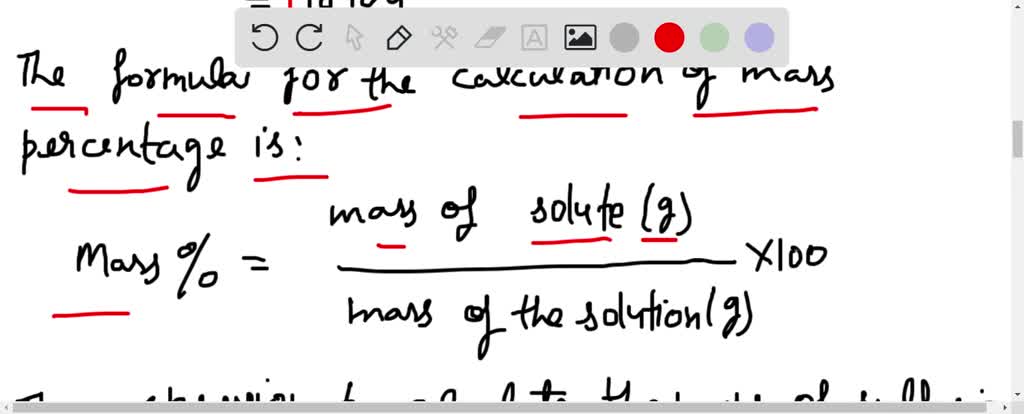
SOLVED:Sulfuric acid has a great affinity for water, and for this reason, the most concentrated form of sulfuric acid available is actually a 98.3% solution. The density of concentrated sulfuric acid is
What volume of concentrated H2SO4 (density = 1.84g/mL and 96% purity) would be required to prepare 500mL of a 0.2000M H2SO4 solution? - Quora

SOLVED: A chemist needs 33.0 g of concentrated sulfuric acid for an experiment. The density of concentrated sulfuric acid at room temperature is 1.84 g/mL. What volume of the acid is required? (