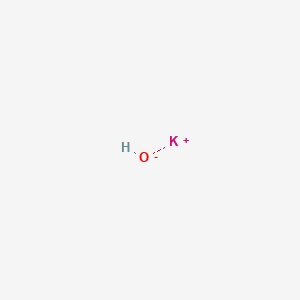SOLVED: What Is the complete ionic, net ionic equation and the spectator ion for the acid- base reaction that occurs when acetic acid and potassium hydroxide solutions are mixed? (4 Points) (d)CH;COO- (

Sulfuric Acid, Hydrochloric Acid, Nitric Acid, Glacial Acetic Acid, Formaldehyde, Formic Acid Supplier

Conductometric titration of a mixture of hydrochloric and acetic acids... | Download Scientific Diagram

What is the mechanism to carvacrol to from 2-(5-isopropyl-2-methylphenoxy) acetic acid by adding potassium hydroxide and chloroacetic acid? | Homework.Study.com












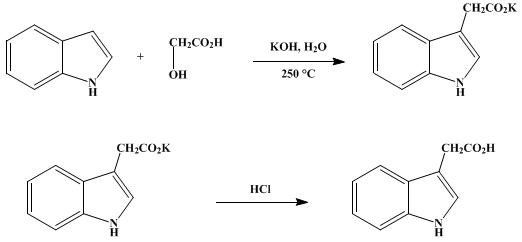
![Synthesis of (A) [[[2-[3-(Dimethylamino)propoxy]phenyl]methylene]amino]-acetic acid, ethyl ester Synthesis of (A) [[[2-[3-(Dimethylamino)propoxy]phenyl]methylene]amino]-acetic acid, ethyl ester](https://prepchem.com/image/substance/5rlochgm3t4404ogk4488s0gwocsskkowk8sgogkw0o08gg044)