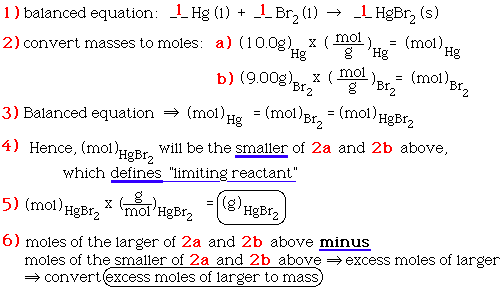Coatings | Free Full-Text | Electro-Oxidation of Ammonia over Copper Oxide Impregnated γ-Al2O3 Nanocatalysts

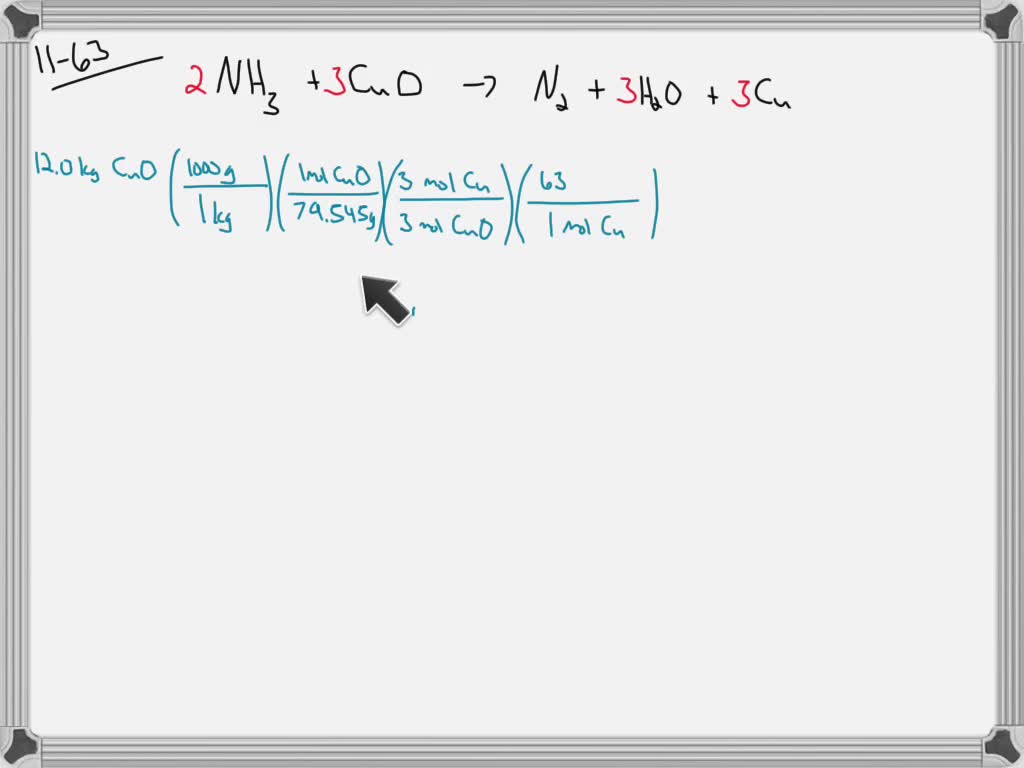
SOLVED: How many moles of nitrogen gas would be produced if 3.40 moles of copper(II) oxide were reacted with excess ammonia in the following chemical reaction? 2 NHz(g) + 3 CuO (s) -
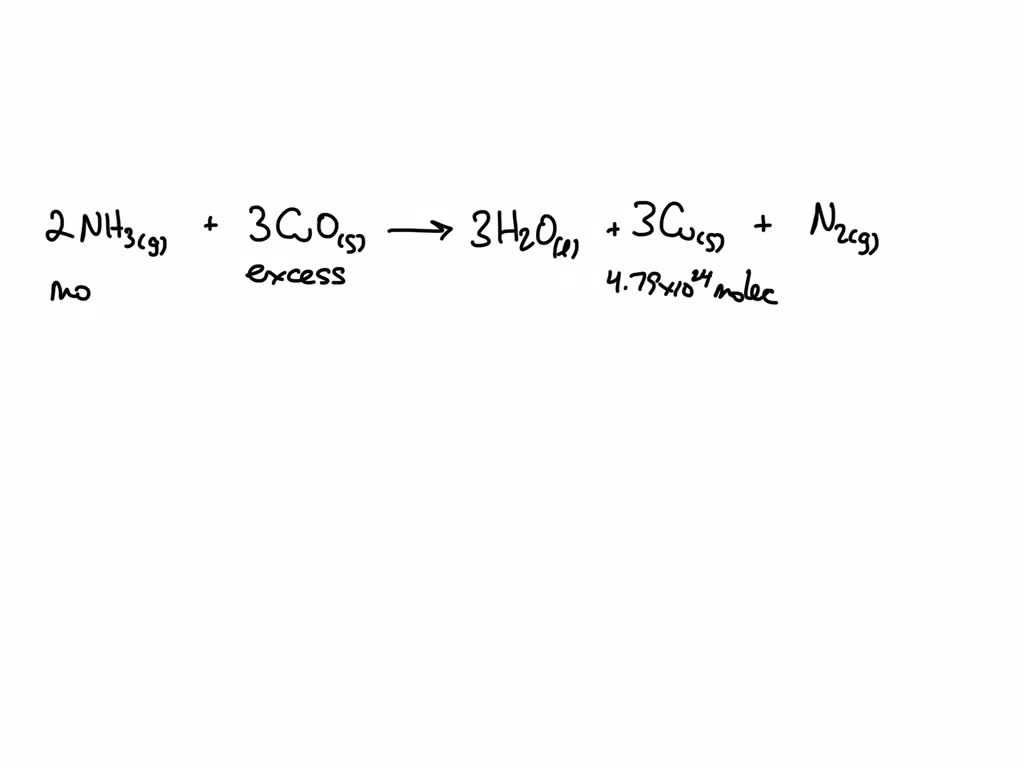
SOLVED: Ammonia gas and solid copper(II) oxide react to form liquid water, solid copper, and nitrogen gas. If copper(II) oxide is present in excess, how many moles of ammonia are needed to

Coatings | Free Full-Text | Electro-Oxidation of Ammonia over Copper Oxide Impregnated γ-Al2O3 Nanocatalysts
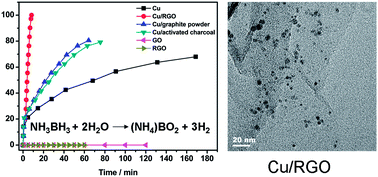
Facile in situ synthesis of copper nanoparticles supported on reduced graphene oxide for hydrolytic dehydrogenation of ammonia borane - RSC Advances (RSC Publishing)
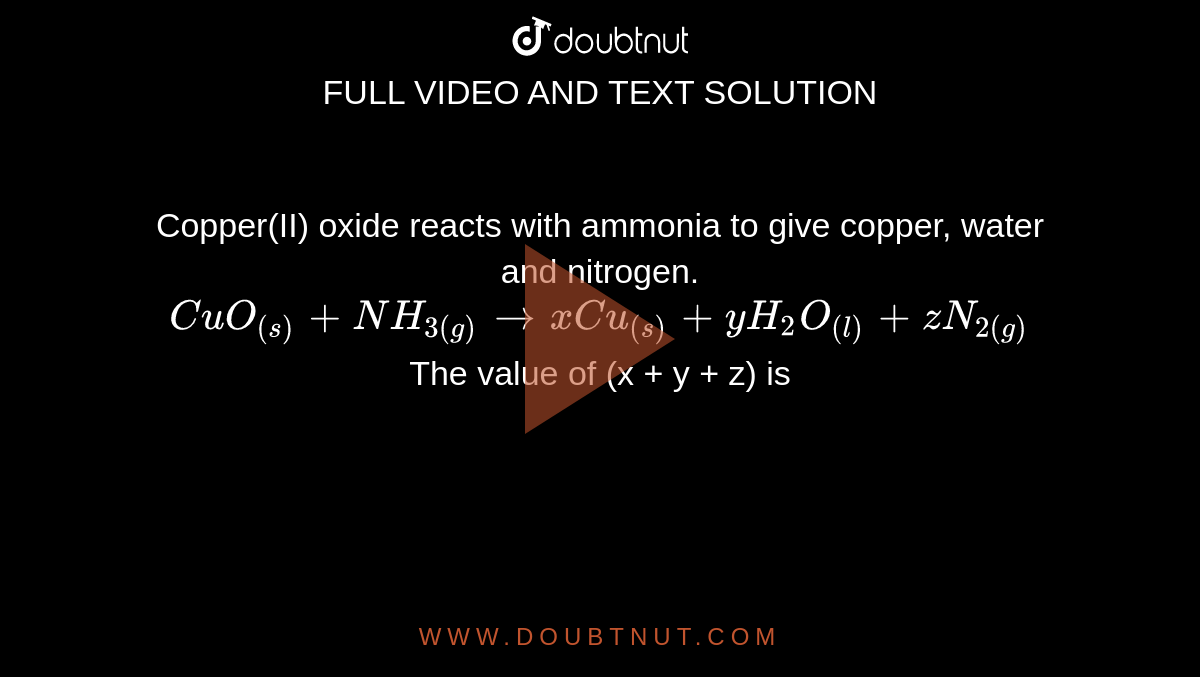
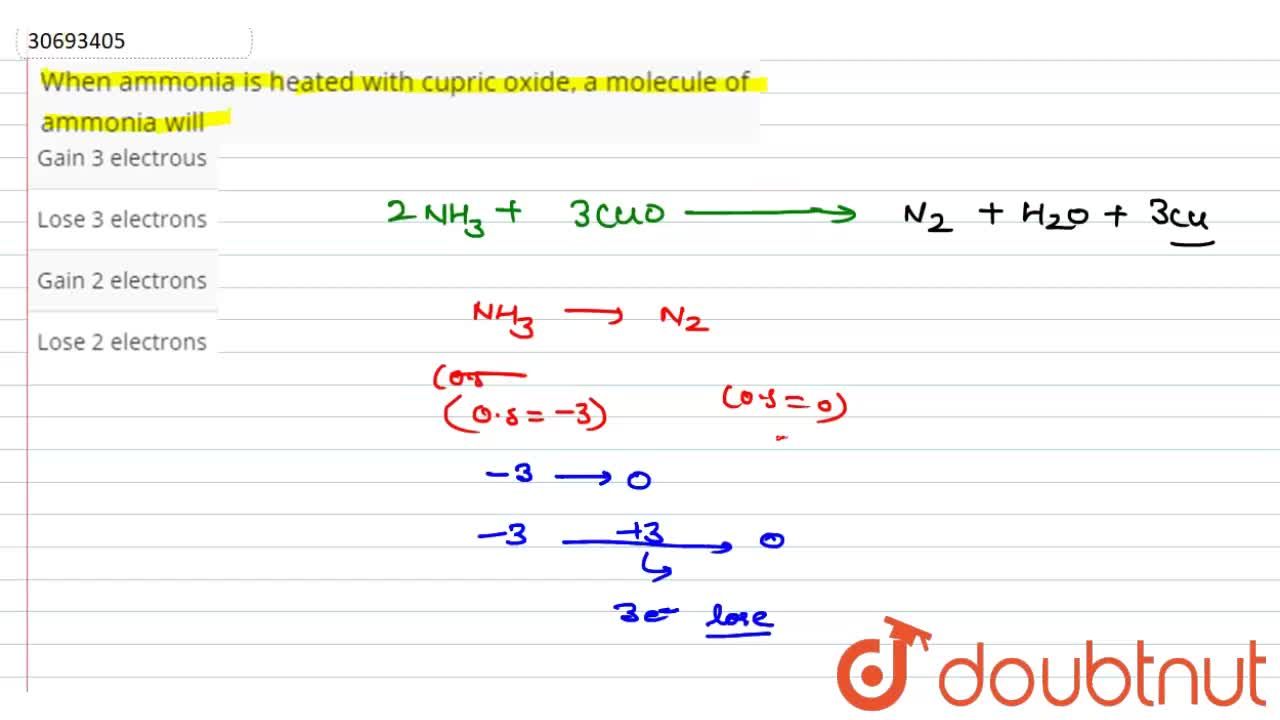
Copper(II) oxide reacts with ammonia to give copper, water and nitrogen. CuO((s)) +NH(3(g)) to xCu((s))+yH(2)O((l)) +zN(2(g)) The value of (x + y + z) is

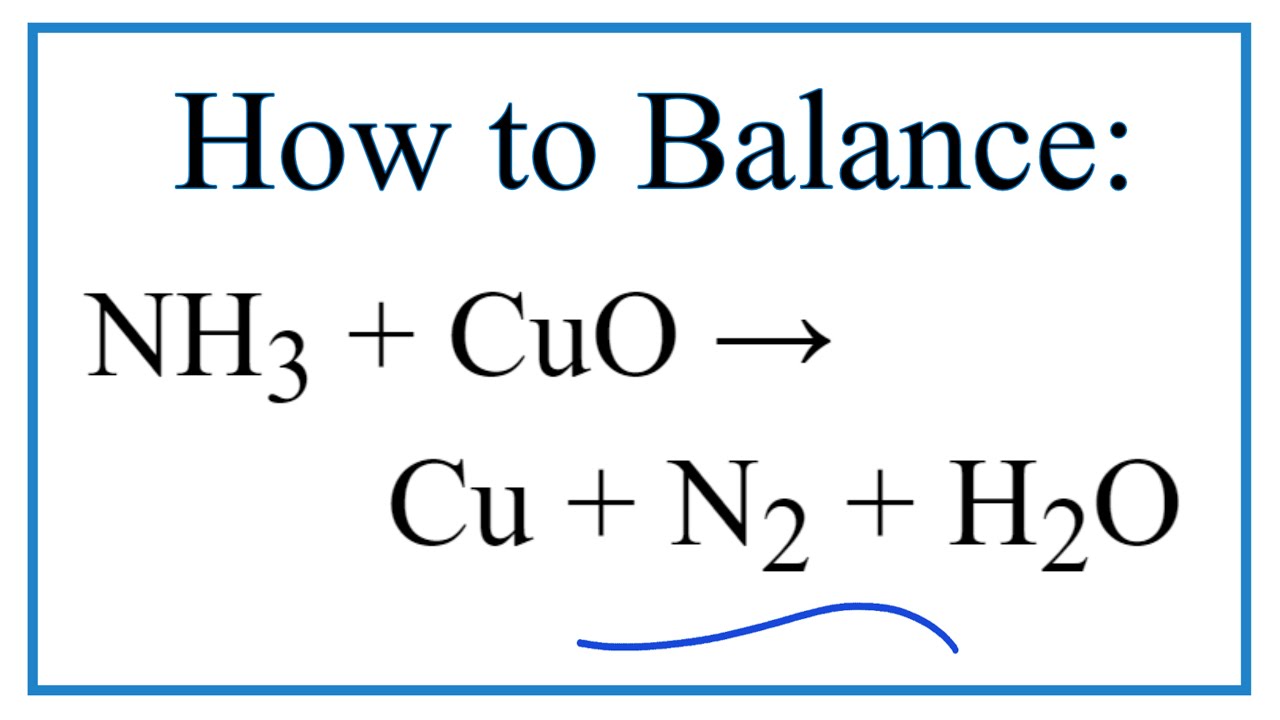
When ammonia is passed over heated copper oxide, the metallic copper is obtained, the reaction shows - YouTube

Give balanced equation for each of the following:Reduction of hot Copper (II) oxide to copper using ammonia gas:
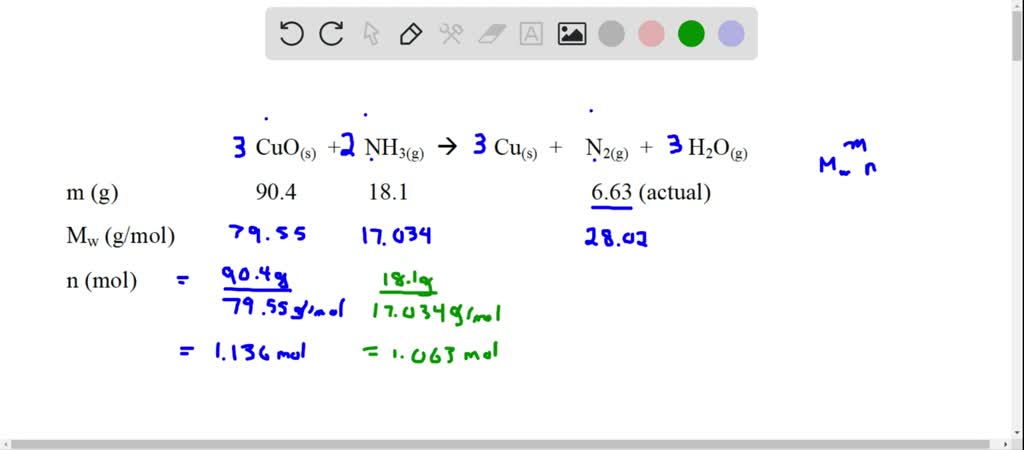
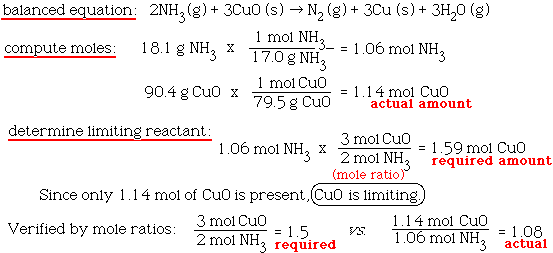
SOLVED: Gaseous ammonia, NH3, and copper(II) oxide, CuO, react together to form gaseous nitrogen (N2), solid copper, and water vapor. If a sample containing 17.9 g of NH3is reacted with 90.0 g

Activating copper oxide for stable electrocatalytic ammonia oxidation reaction via in-situ introducing oxygen vacancies | SpringerLink

When ammonia is passed over heated copper oxide, the metallic copper is obtained, the reaction shows - YouTube

Selective catalytic oxidation of ammonia to nitric oxide via chemical looping | Nature Communications