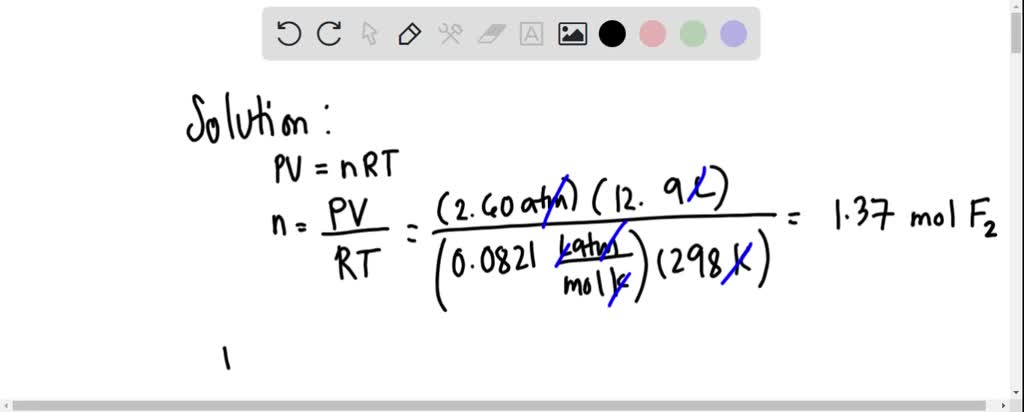
SOLVED:During the time 0.305 mol of an ideal gas undergoes an isothermal compression at 22.0^∘ C, 392 J of work is done on it by the surroundings. (a) If the final pressure
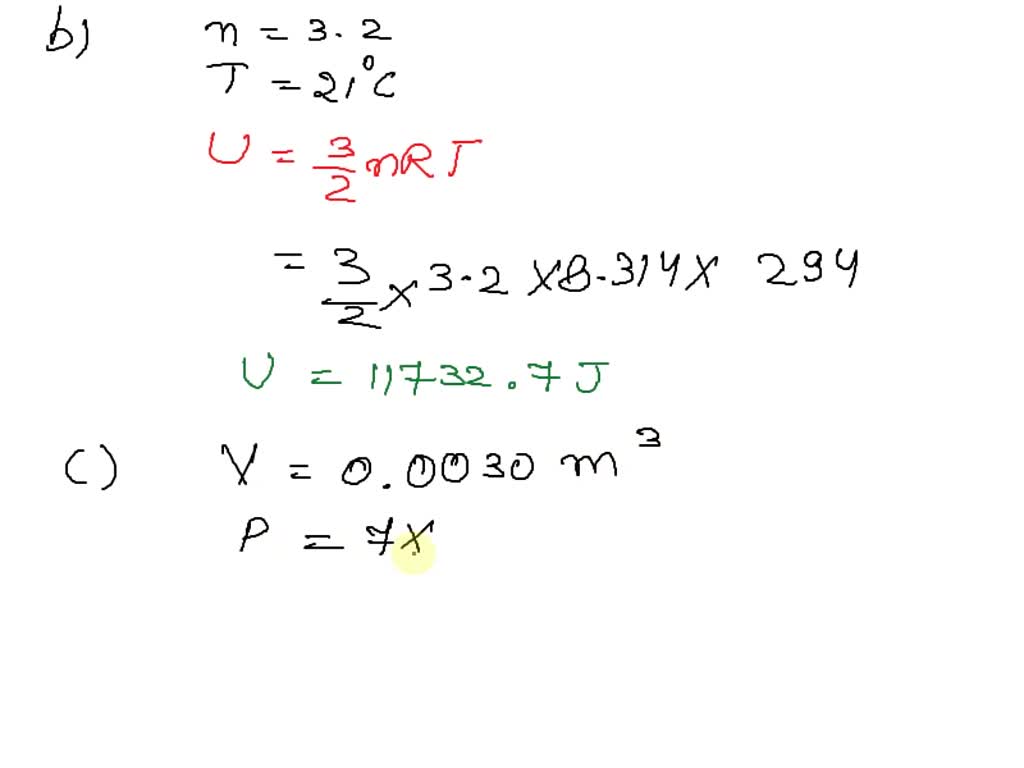
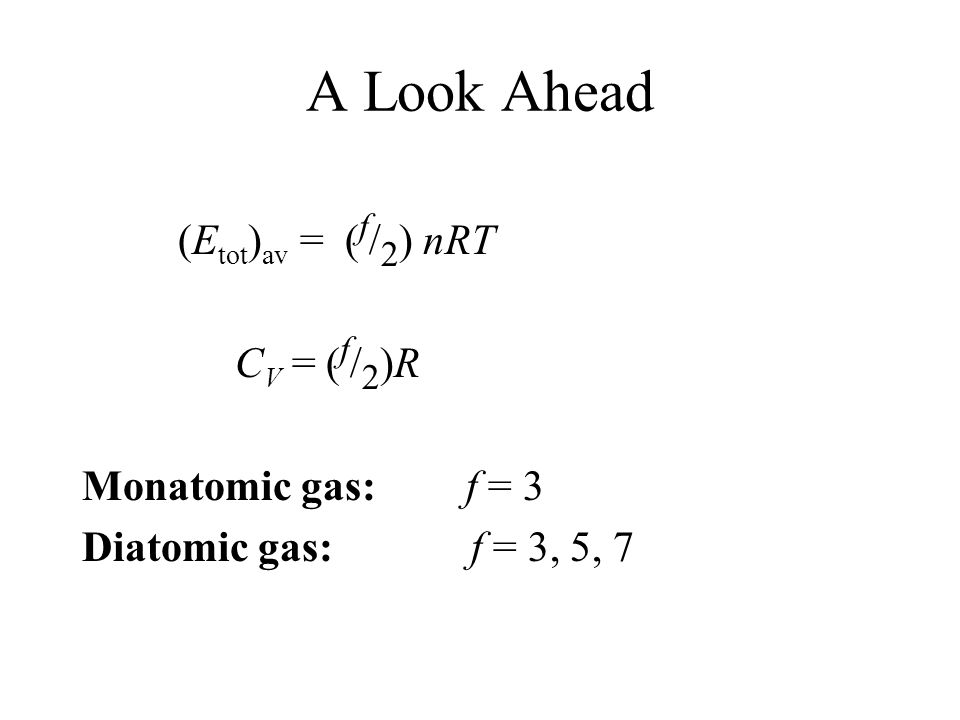
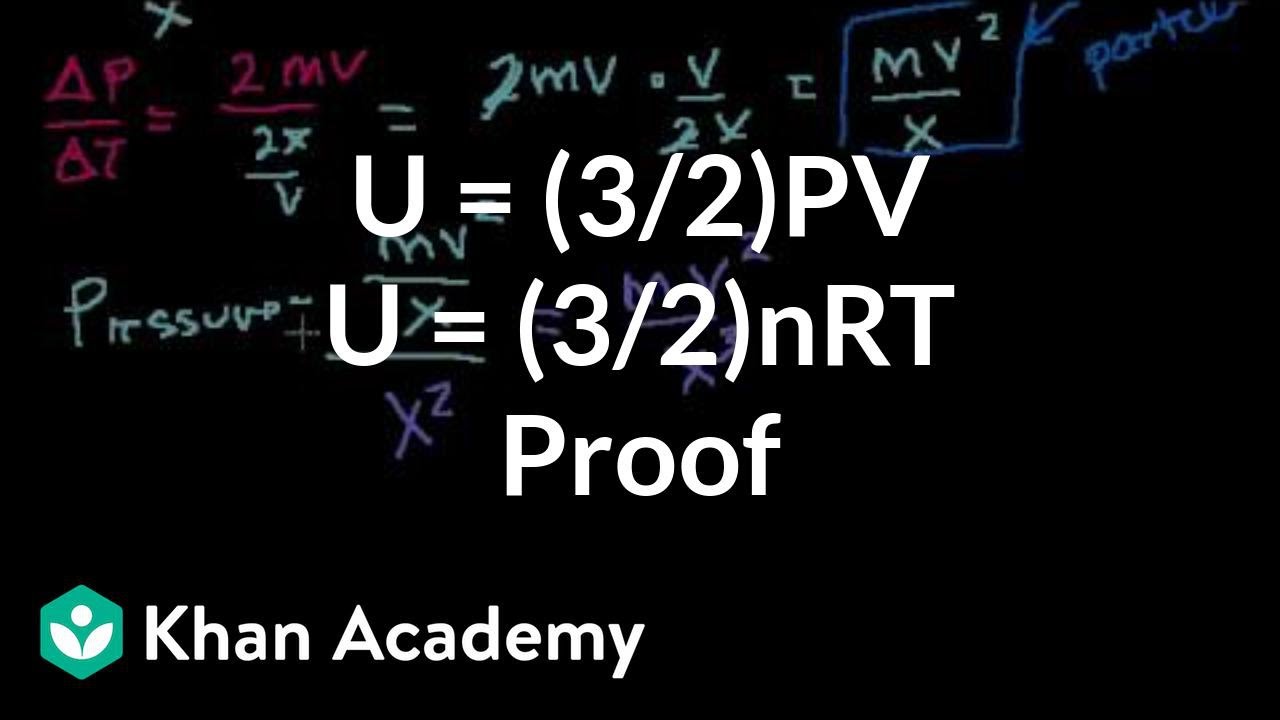
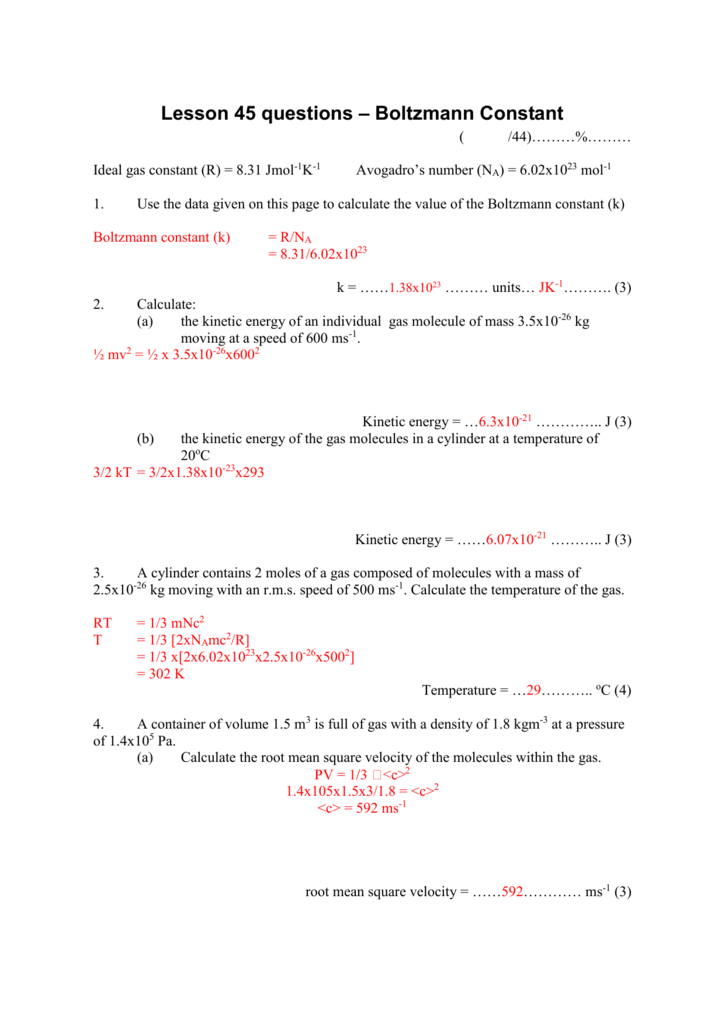
SOLVED: Ine average Kinetic energy of molecule; I5 called thermal energy; itiS directly related to absolute temperature. KE (average per molecule) m v2(average) ket (KB =1.38*10-23 VK) Jkb 2. The average speed
Sustainability | Free Full-Text | Experiential Graduate Course Prepares Transdisciplinary Future Leaders to Innovate at the Food-Energy-Water Nexus

SOLVED: Kinematits: Dx = vt Ax = Vot +%2 v =Vo v2 v3 = Zafx Ax = () (vo v)t 9.80 [Vs? v =" ac = v/r Dynamics: mu = 2F max
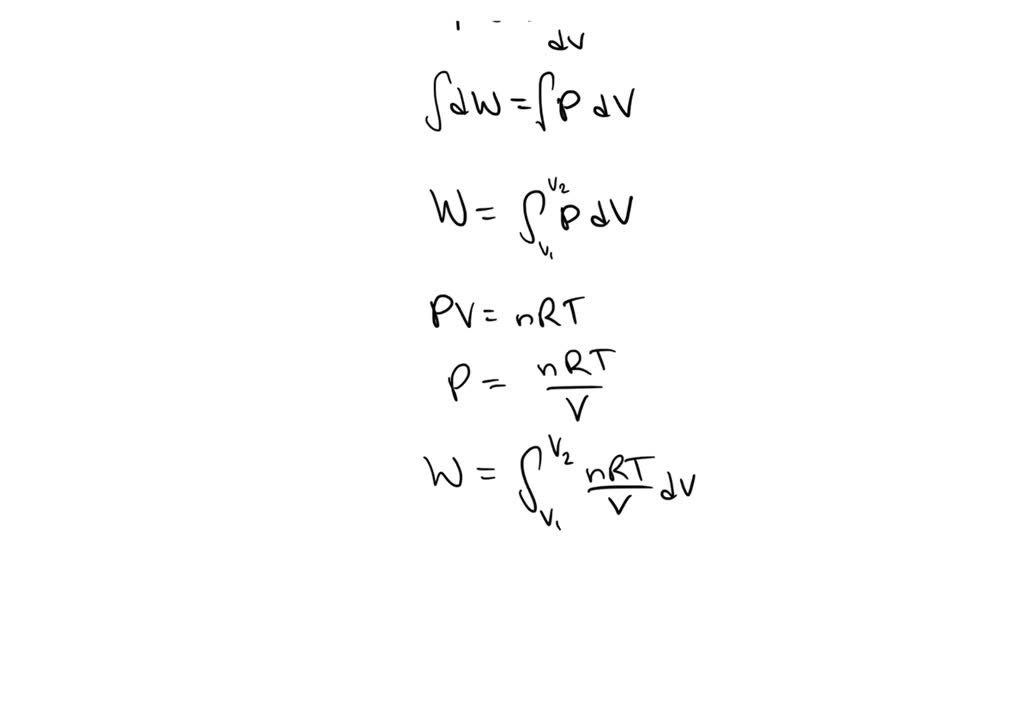
SOLVED: A quantity of gas with an initial volume of 3 cubic feet and a pressure of 800 pounds per square foot, expands to a volume of 4 cubic feet. Find the
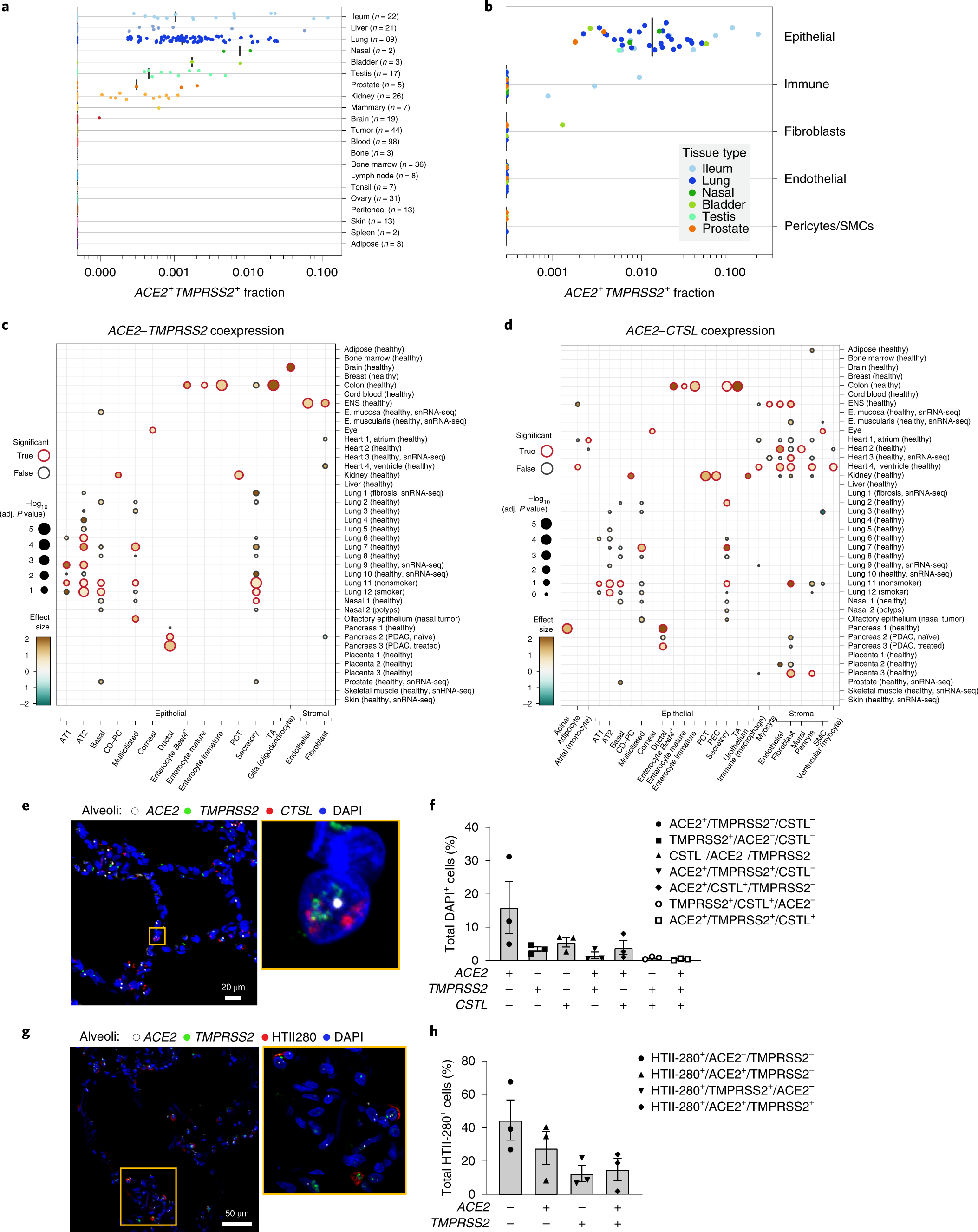
Single-cell meta-analysis of SARS-CoV-2 entry genes across tissues and demographics | Nature Medicine
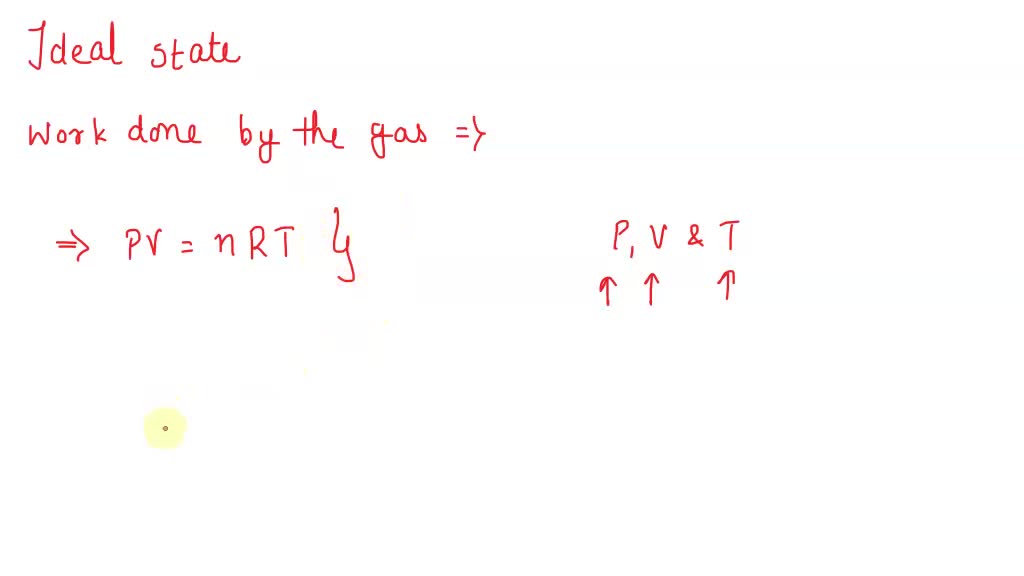
SOLVED: 'All of the following are factors directly affecting the work by an ideal gas except: pressure in the initial and final state heat transfer in the initial and final state thermodynamic
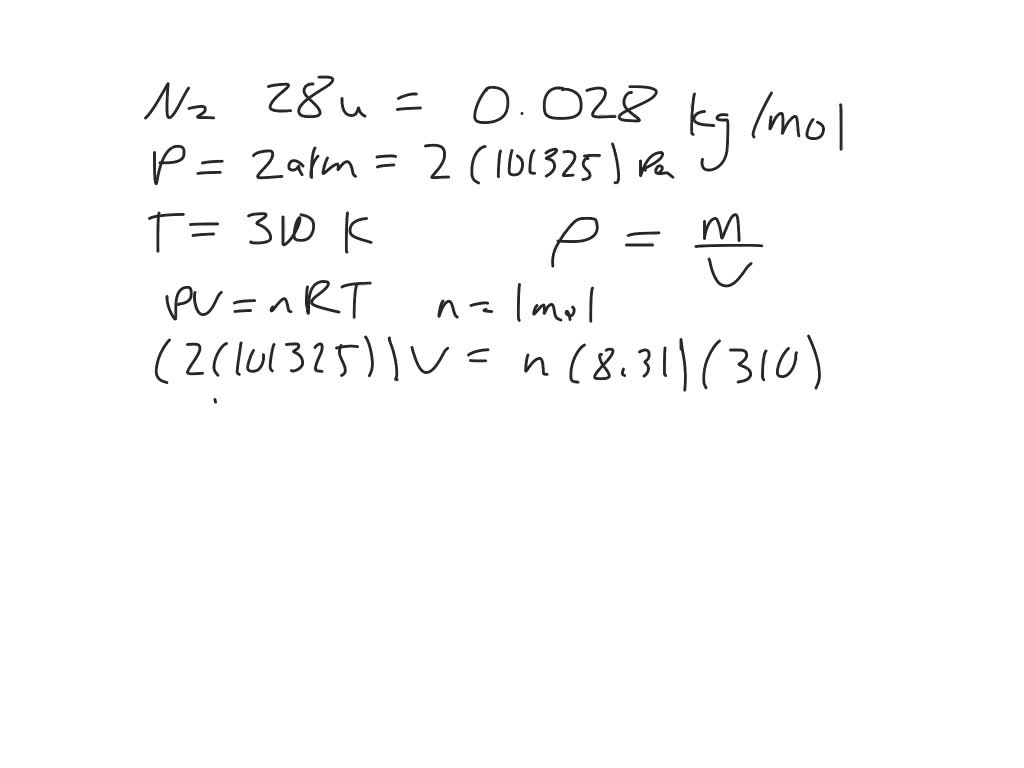
SOLVED:ssm What is the density (in kg / m^3 ) of nitrogen gas (molecular mass =28 u ) at a pressure of 2.0 atmospheres and a temperature of 310 K ?