
If you have 500 mL of 0.15 M formic acid, what is the pH of this solution? What is the pKa? How many grams of sodium formate would you have to add

The comparison of pKa determination between carbonic acid and formic acid and its application to prediction of the hydration numbers - ScienceDirect

OneClass: What is the necessary ratio of potassium formate to formic acid to make a solution of pH = ...
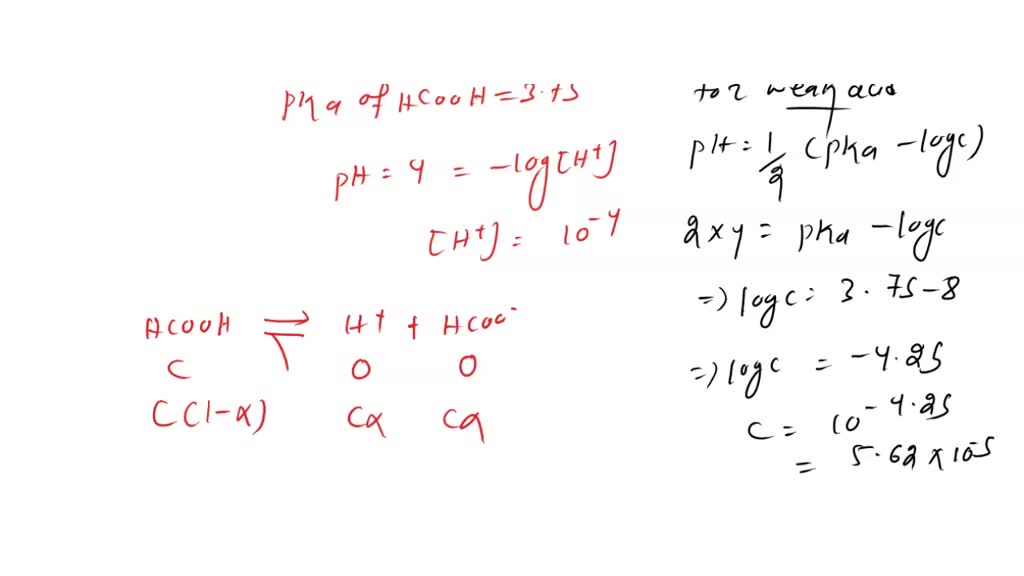
SOLVED: Formic acid (HCOOH) has a pKa of 3.75. (a) What percent of formic acid dissociates at pH 4? (b) What pH will the ratio of formic acid to formate be 2:1?
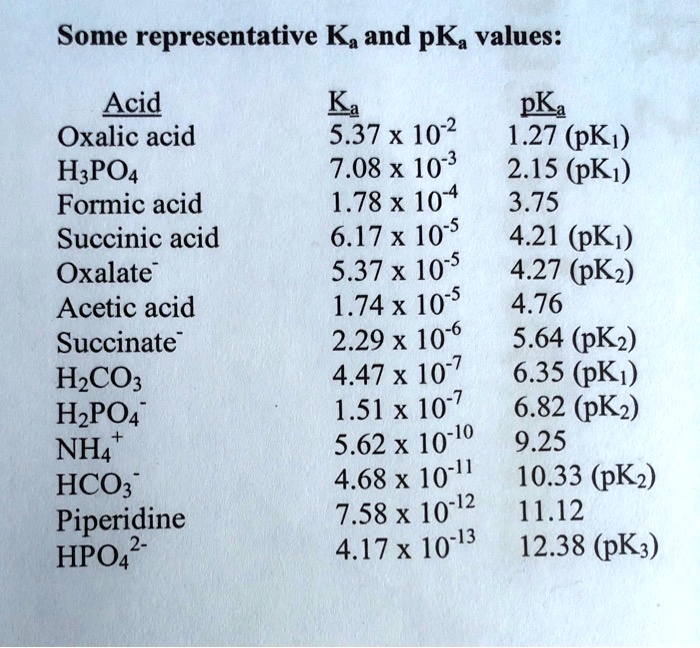
SOLVED: Some representative Ka and pKa values: Acid Oxalic acid HzPOa Formic acid Succinic acid Oxalate Acetic acid Succinate HzCOz HzPOA NH4' HCO; Piperidine HPOA2 Ka pKa 5.37 x 10-2 1.27 (pK,)

If you have 500 mL of 0.15 M formic acid, what is the pH of this solution? What is the pKa? How many grams of sodium formate would you have to add
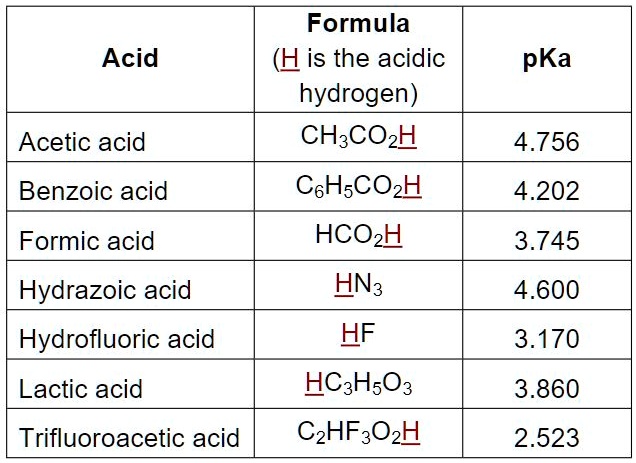
SOLVED: Formula Acid (His the acidic hydrogen) CH:COzH pKa Acetic acid 4.756 Benzoic acid CsHsCOzH HCOzH HN3 HF 4.202 Formic acid 3.745 Hydrazoic acid Hydrofluoric acid Lactic acid 4.600 3.170 HCHsO3 3.860 Trifluoroacetic acid CzHF3OzH 2.523

What is the pH of a 0.15 M solution of formic acid, HCOOH ? `{:("Formic Acid ",K_a),(HCOOH - YouTube


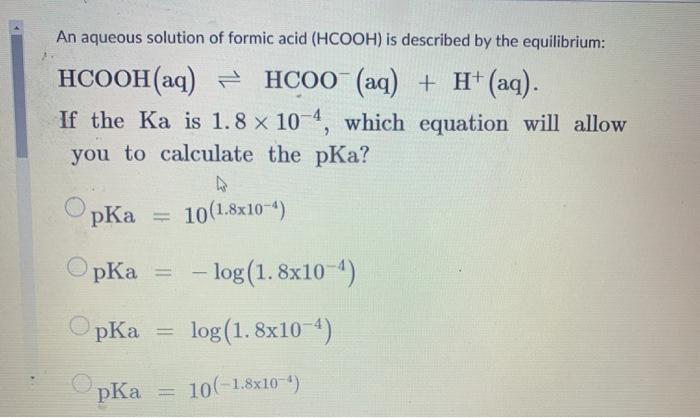
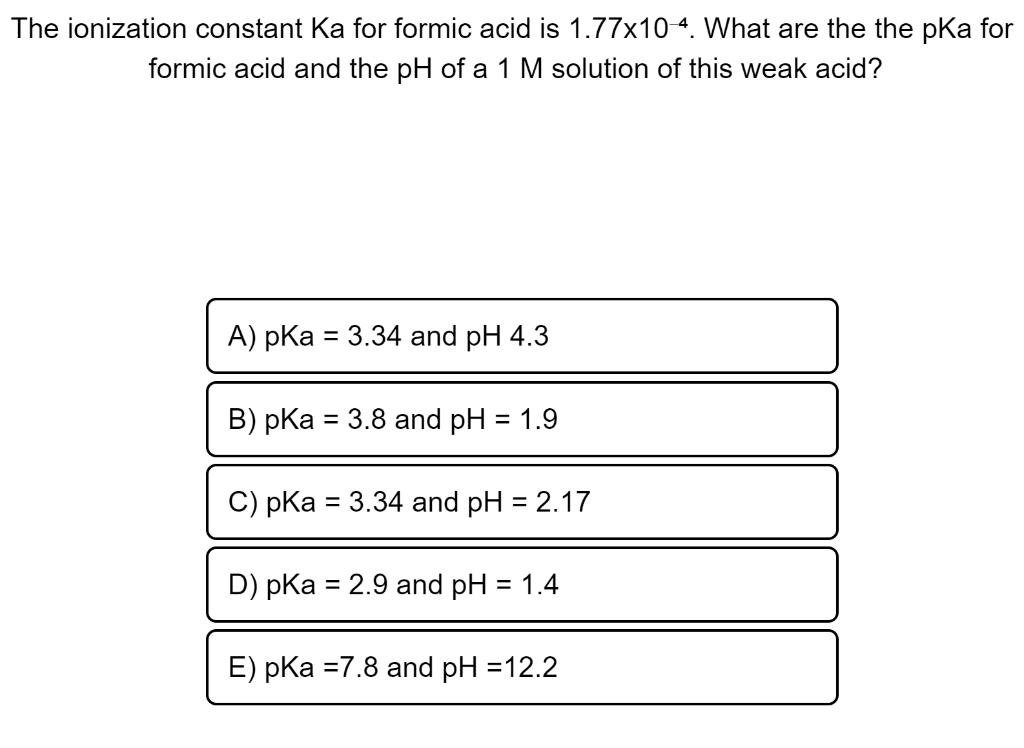



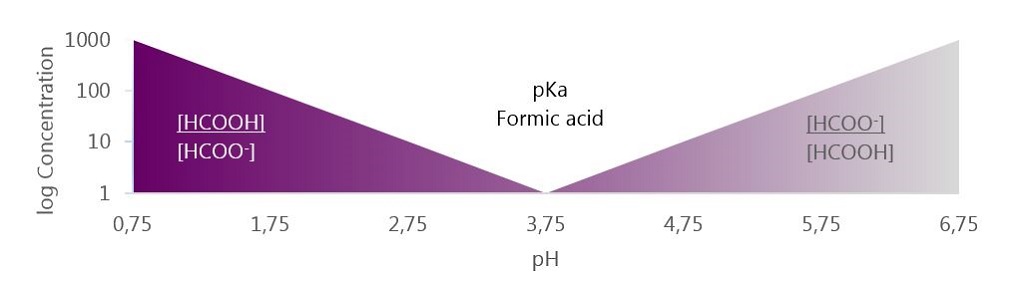

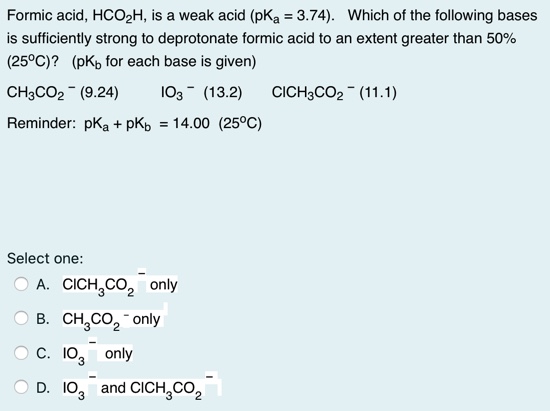
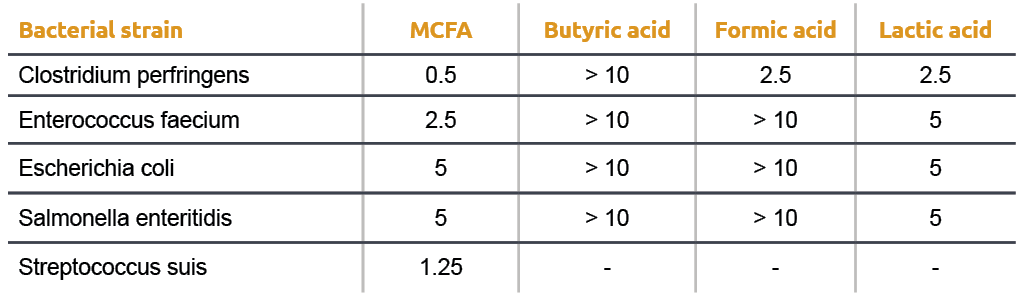
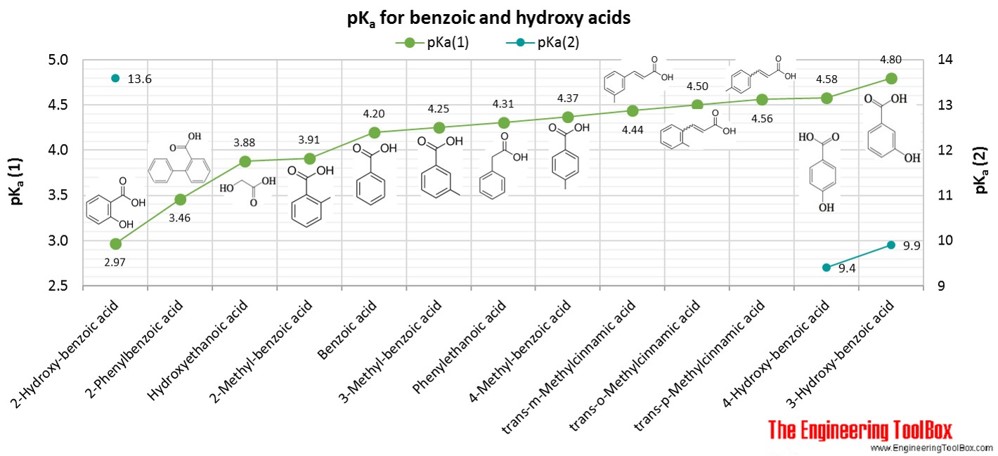

![Solved What is the ratio [A]/[HA] at pH 3.75? The pKa of | Chegg.com Solved What is the ratio [A]/[HA] at pH 3.75? The pKa of | Chegg.com](https://media.cheggcdn.com/media/5a3/5a32fe0d-21ca-4cf0-9a37-a59affbc86ba/phpMgWDCv.png)
