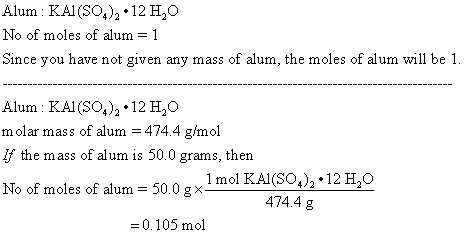Cubic structure of alum K(Al,Cr)(SO 4 ) 2 ⋅12H 2 O, space group Pa3 _ .... | Download Scientific Diagram

Kal(so4)2.12h2o Aluminium Potassium Sulfate Potassium Alum Powder - Buy Potassium Alum Powder,Aluminium Potassium Sulfate Powder,Kal(so4)2 12h2o Potassium Alum Product on Alibaba.com
![Table I from Alum [KAl(SO4)2·12H2O]: An Efficient, Novel, Clean, Catalyst for Doebner—Knoevenagel Reaction for the Efficient Production of α,β‐Unsaturated Acids. | Semantic Scholar Table I from Alum [KAl(SO4)2·12H2O]: An Efficient, Novel, Clean, Catalyst for Doebner—Knoevenagel Reaction for the Efficient Production of α,β‐Unsaturated Acids. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/f3ee51bfb8cf7b09279205adbb73911287275c36/2-TableI-1.png)
Table I from Alum [KAl(SO4)2·12H2O]: An Efficient, Novel, Clean, Catalyst for Doebner—Knoevenagel Reaction for the Efficient Production of α,β‐Unsaturated Acids. | Semantic Scholar

SOLVED: KAl(SO4)2 reacts with BaCl2 in aqueous solutions to produce a precipitate of BaSO4 that can be filtered and weighed. The balanced equation for the reaction is: KAl(SO4)2 (aq) + 2 BaCl2 (

Suppose Harry begins with the hydrate KAl(SO4)2·12H2O. After dehydration he finds that he is left - Brainly.com
Thermal behaviour of alum-(K) KAl(SO4)2·12H2O from in situ laboratory high-temperature powder X-ray diffraction data: thermal e

Kal(so4)2 12h2o Aluminum Potassium Sulfate Edible Potassium Alum - Buy Kal( so4)2 12h2o Potassium Alum,Edible Potassium Alum,Aluminum Potassium Sulfate Product on Alibaba.com

Kal(so4)2 12h2o Aluminum Potassium Sulfate Lump Potassium Alum Potash Alum - Buy Potash Alum,Aluminum Potassium Sulfate,Kal(so4)2 12h2o Potash Alum Product on Alibaba.com
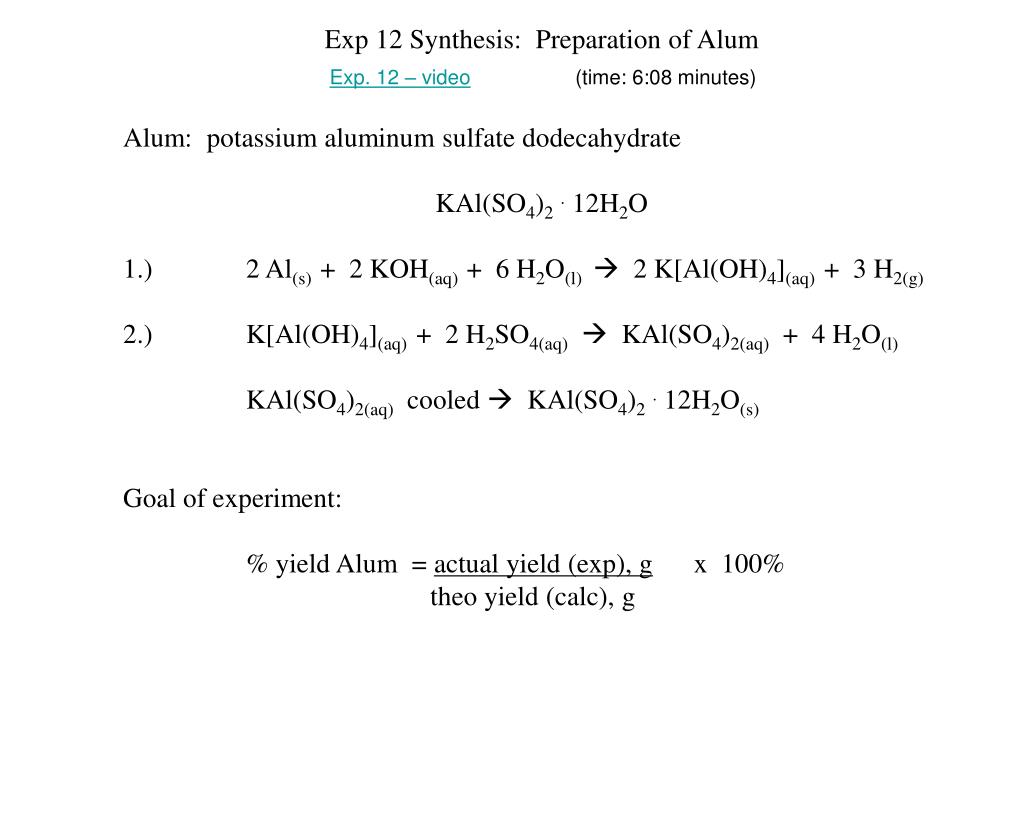
PPT - Exp 12 Synthesis: Preparation of Alum Alum: potassium aluminum sulfate dodecahydrate PowerPoint Presentation - ID:5409973
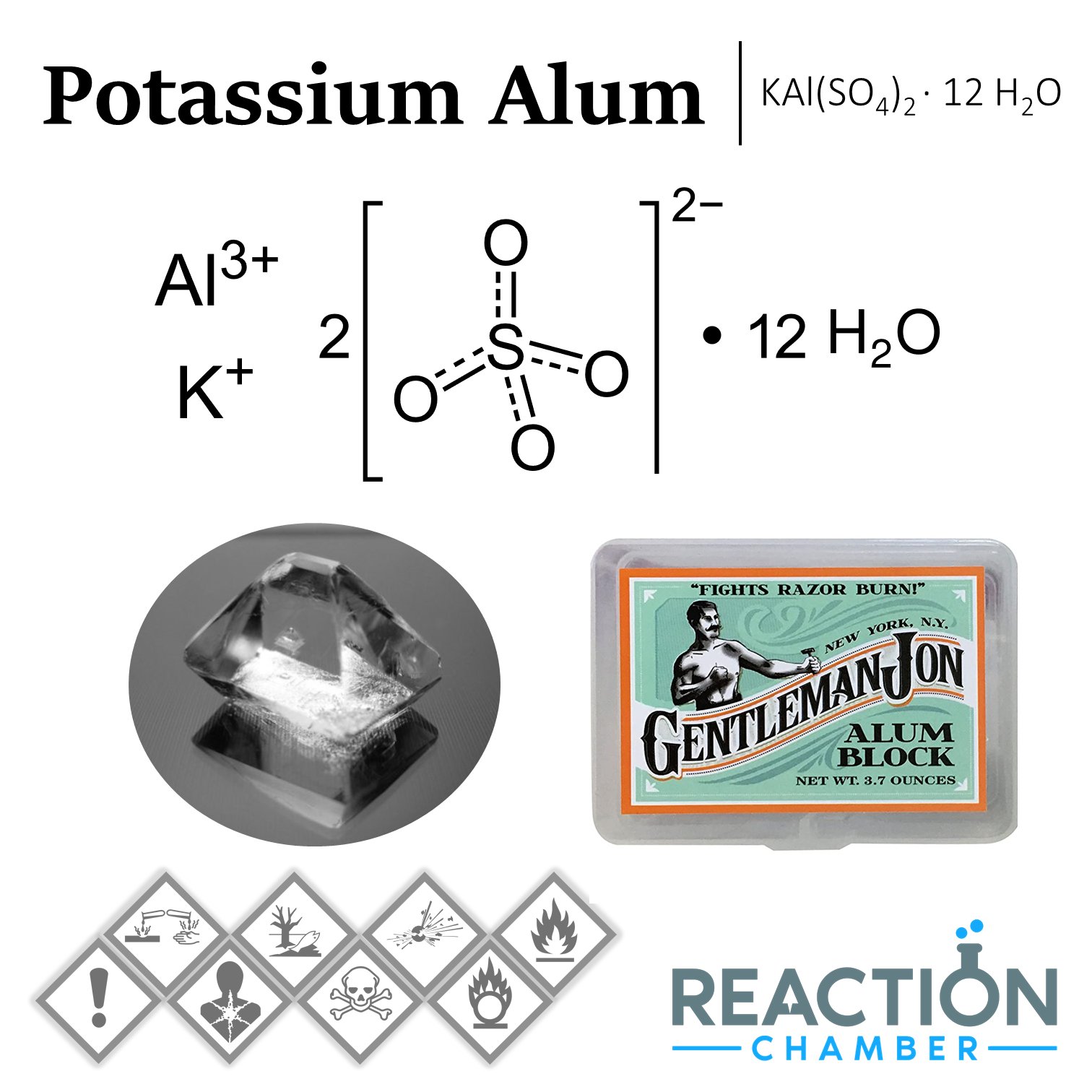
reactionchamber on Twitter: "Potassium alum is the double sulfate salt of potassium and aluminium. It is commonly encountered as the dodecahydrate, KAl(SO4)2·12H2O. It crystallizes in an octahedral structure in neutral solution. #alum #