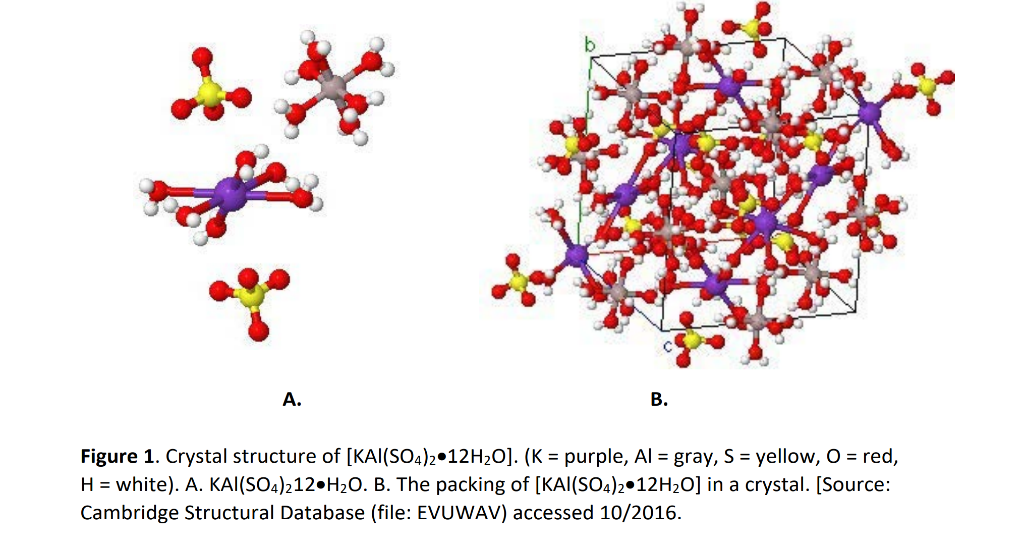
![Potassium Alum [KAl(SO4)2∙12H2O] solid catalyst for effective and selective methoxylation production of alpha-pinene ether products - ScienceDirect Potassium Alum [KAl(SO4)2∙12H2O] solid catalyst for effective and selective methoxylation production of alpha-pinene ether products - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S2405844021001638-sc1.jpg)
Potassium Alum [KAl(SO4)2∙12H2O] solid catalyst for effective and selective methoxylation production of alpha-pinene ether products - ScienceDirect
Why do alum (potassium aluminum) crystals make octahedral shapes? What is the shape of the molecule? - Quora

Cubic structure of alum K(Al,Cr)(SO 4 ) 2 ⋅12H 2 O, space group Pa3 _ .... | Download Scientific Diagram
Potassium Alum [KAl(SO4)2∙12H2O] solid catalyst for effective and selective methoxylation production of alpha-pinene

Polarized Raman Spectra of NH𝟒Al(SO𝟒)𝟐⋅𝟏𝟐H𝟐O – topic of research paper in Nano-technology. Download scholarly article PDF and read for free on CyberLeninka open science hub.
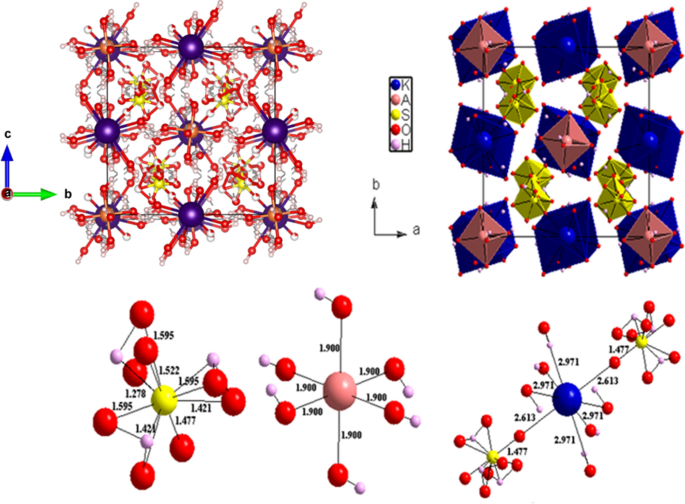
Investigations on KAl(SO4)2∙12H2O: A Candidate α-Alum Material for Energy Storage Applications | SpringerLink
A study of the relaxation mechanism of a KAl(SO4)2·12H2O single crystal by observation of its 39K and 27Al spin–lattice relax
Thermal behaviour of alum-(K) KAl(SO4)2·12H2O from in situ laboratory high-temperature powder X-ray diffraction data: thermal e
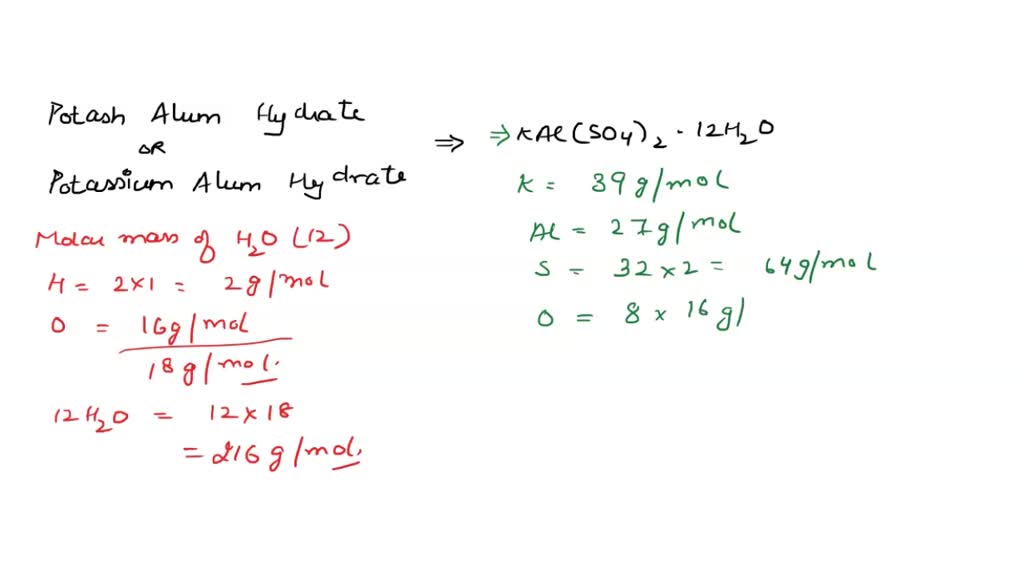
SOLVED: What is the mass percent of water in the Potassium Alum Hydrate: KAl (SO4)2·12H2O? A) 8.33% B) 91.67% C) 21.62% D) 54.43% E) 45.57%
Why do alum (potassium aluminum) crystals make octahedral shapes? What is the shape of the molecule? - Quora

Question Video: Calculating the Percentage by Mass of Water in Alum Given Its Chemical Formula | Nagwa

Investigations on KAl(SO4)2∙12H2O: A Candidate α-Alum Material for Energy Storage Applications | SpringerLink
Crystal Chemistry and High-Temperature Behaviour of Ammonium Phases NH4MgCl36H2O and (NH4)2Fe3+Cl5H2O from the Burned Dumps of t
reactionchamber on Twitter: "Potassium alum is the double sulfate salt of potassium and aluminium. It is commonly encountered as the dodecahydrate, KAl(SO4)2·12H2O. It crystallizes in an octahedral structure in neutral solution. #alum #