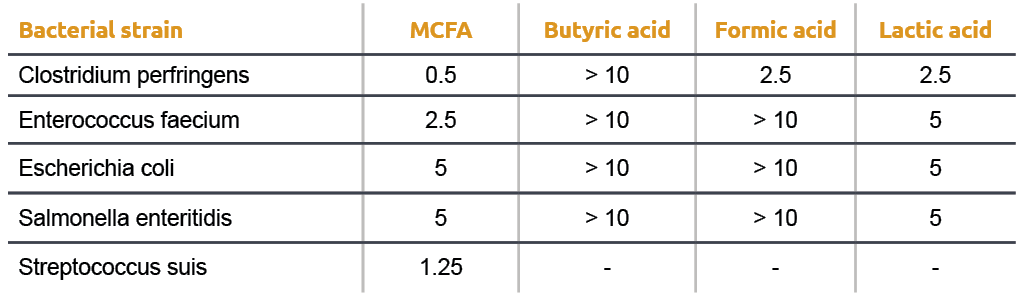
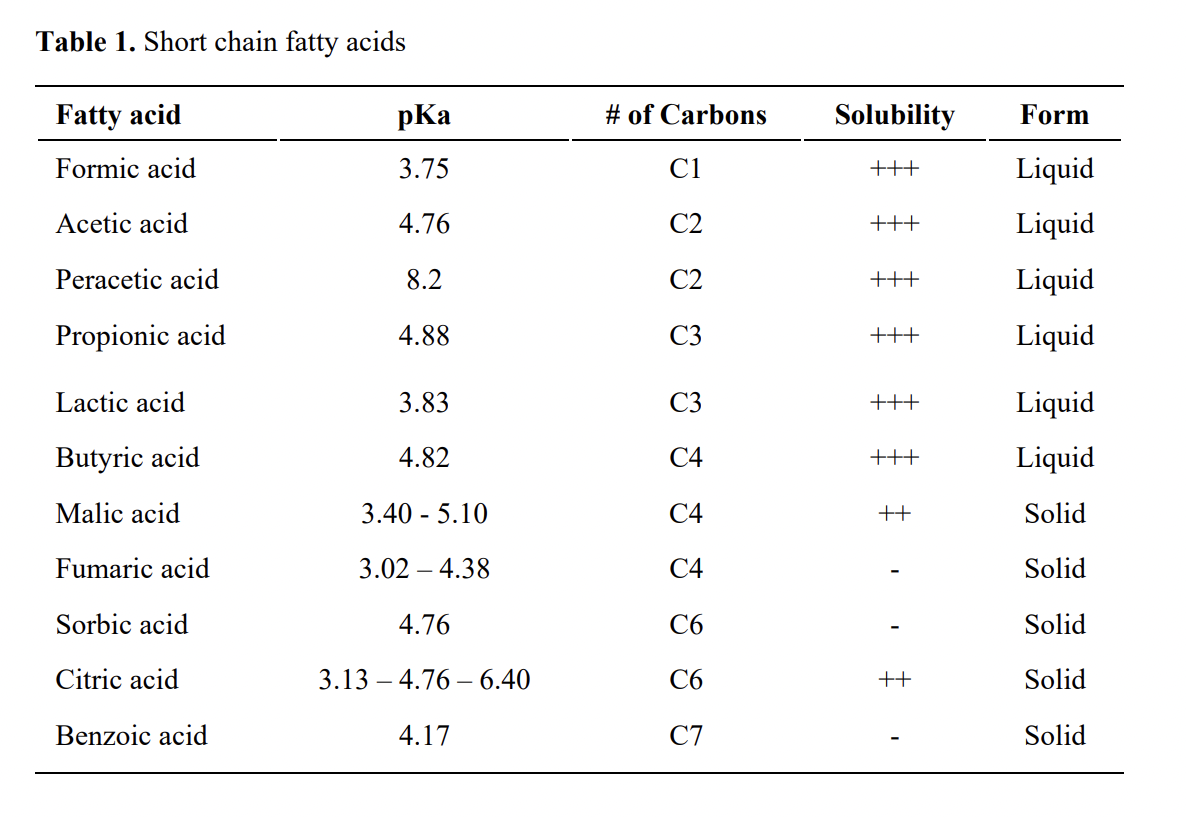
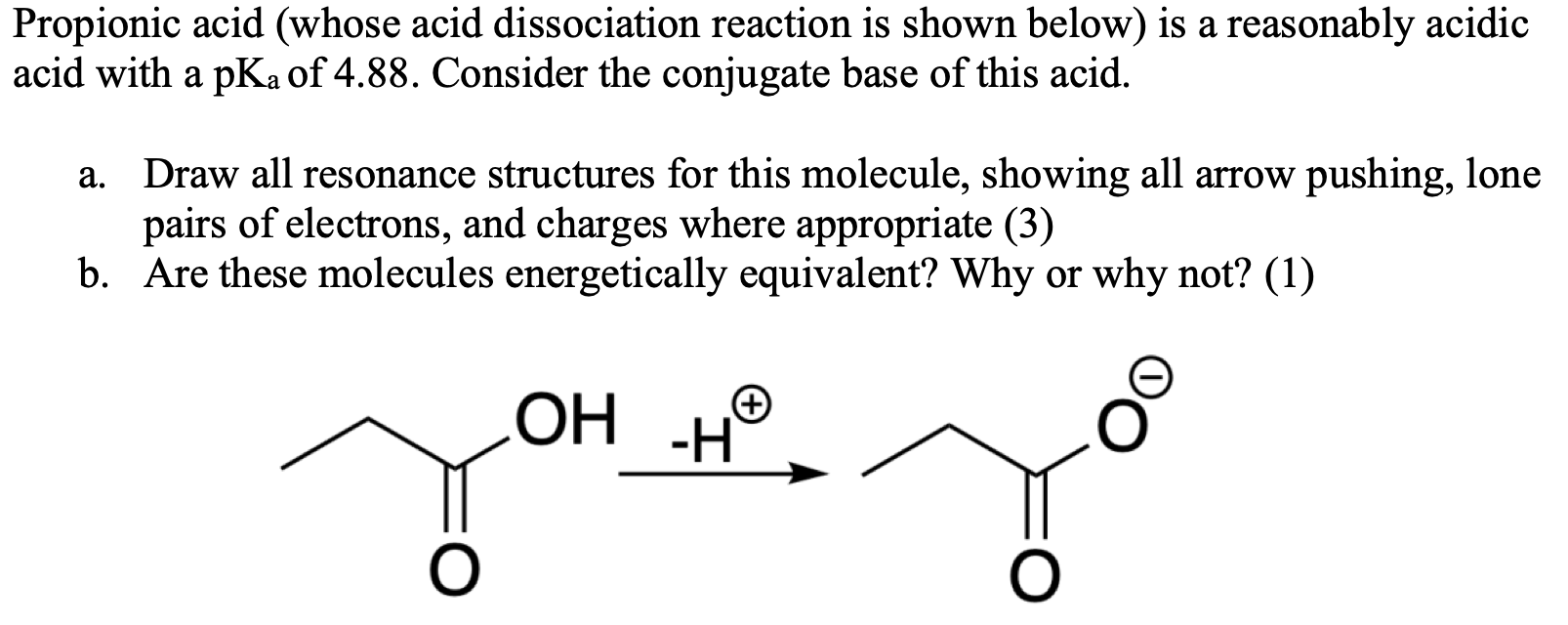
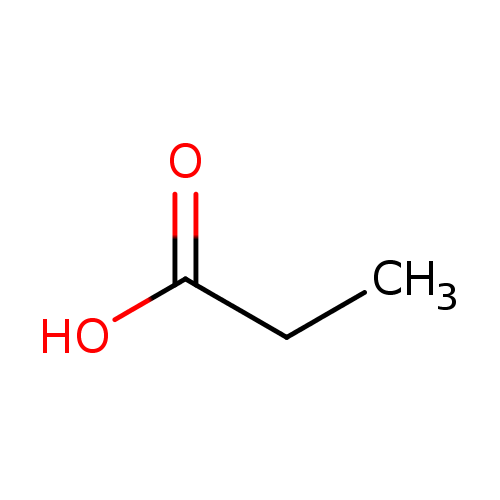
Why is the pka of vanillin lower than salicylic aldehyde, which is lower than the pka of a carboxylic acid (ex: propionic acid)? : r/OrganicChemistry
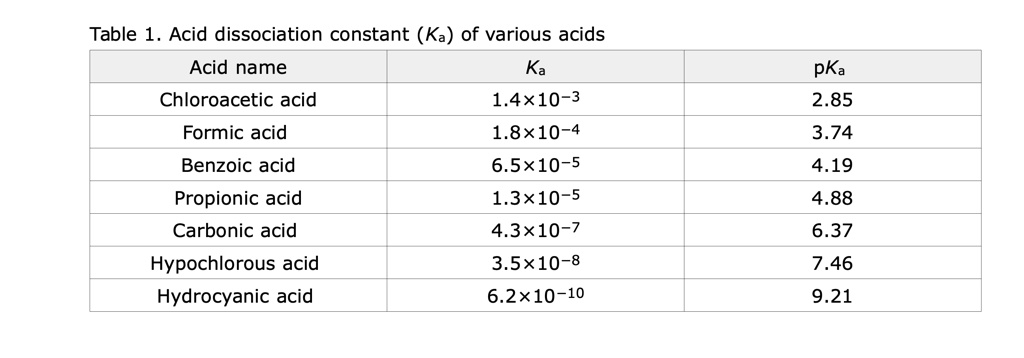
SOLVED: Table Acid dissociation constant (Ka) of various acids Acid name Ka Chloroacetic acid 1.4x10-3 Formic acid 1.8*10-4 Benzoic acid 6.5x10-5 Propionic acid 1.3x10-5 Carbonic acid 4.3*10-7 Hypochlorous acid 3.5x10-8 Hydrocyanic acid

WO2008137496A1 - Compositions for reducing, ameliorating, treating, or preventing condition of dry eye and methods of making and using same - Google Patents

Empirical Conversion of pKa Values between Different Solvents and Interpretation of the Parameters: Application to Water, Acetonitrile, Dimethyl Sulfoxide, and Methanol | ACS Omega

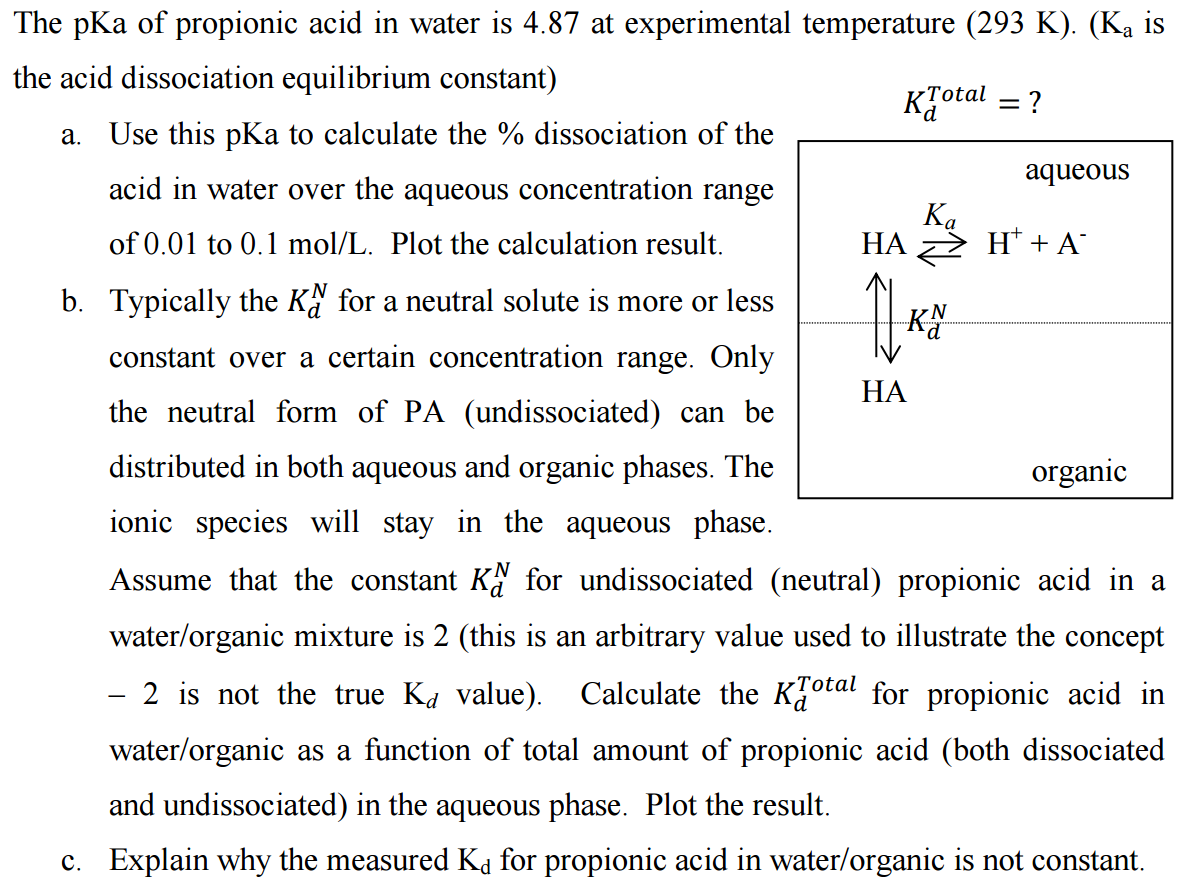
SOLVED: pKa of propionic acid is 4.87. What is the pH of a buffer that is 0.24 propionic acid ( H3C- CHs-COOH) and 0.29 M sodium propanate (H3C-CH2-COONa).
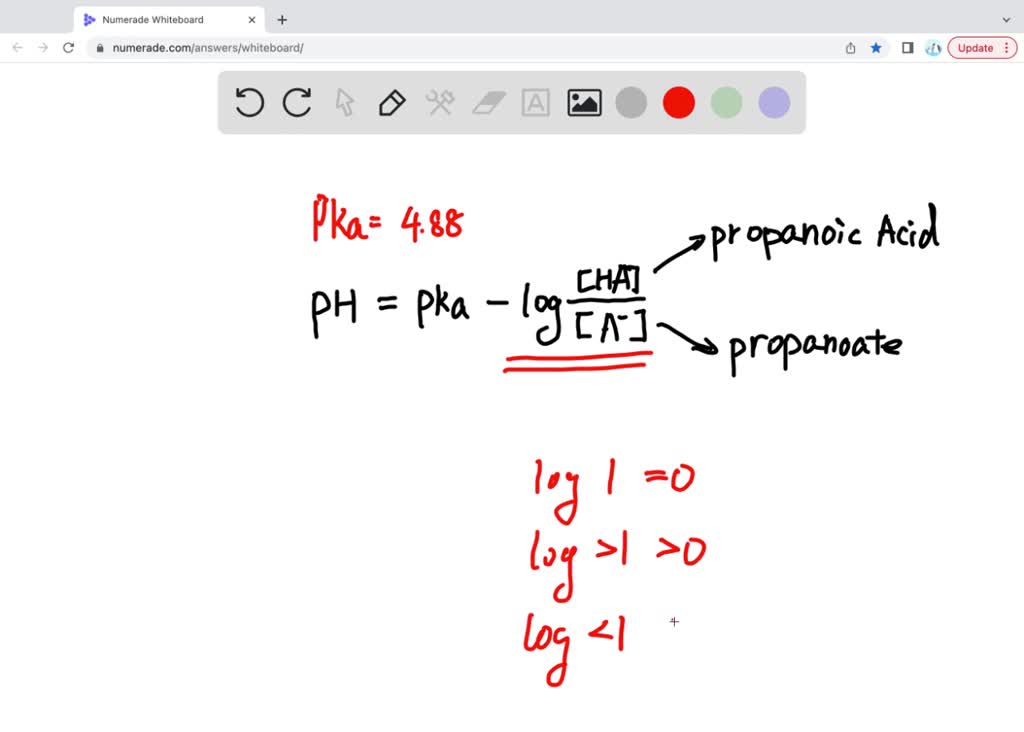
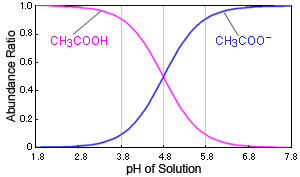
SOLVED: 'The pKa of propanoic acid is 4.88. Describe what the relative concentrations of propanoic acid (the protonated form of this acid) and propanoate (its conjugate base) will be (1) at; (2)

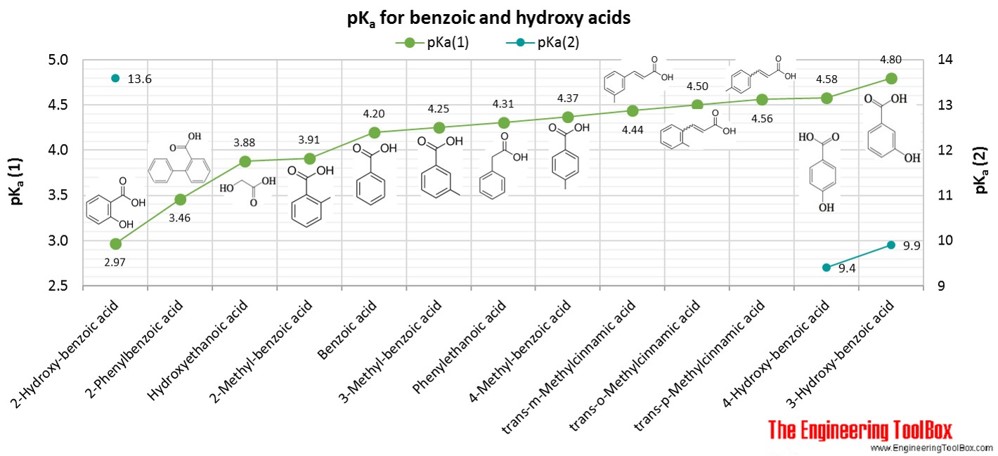


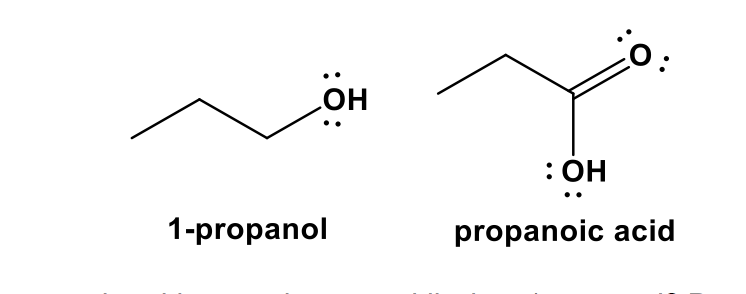


![Solved --] pka of weak acids at 25°C Name Formula рка1 pka2 | Chegg.com Solved --] pka of weak acids at 25°C Name Formula рка1 pka2 | Chegg.com](https://media.cheggcdn.com/media/e5d/e5dd9b94-4668-4e5b-bfb9-43a0c4361419/phpSuOXUG)